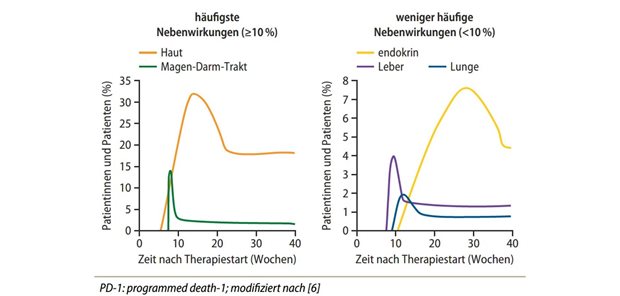
Europe Pharma Agenda: Impulses for Care with Pharmaceuticals in Germany
The term „Unmet Need“ is used multiple times in EU HTA regulations, the EU pharmaceutical legislation and in HTA guidelines. There is no consensus about the definition, yet in most guidance documents it refers to the unavailability of treatment options for patients with severe conditions or for patients suffering from significant residual disease.
Kooperation
|
In Kooperation mit: AbbVie Deutschland, DAK Gesundheit, MSD Sharp & Dohme, Novo Nordisk, Roche Pharma, vfa und Xcenda